癌の治療に新たな光明! 免疫システムによって癌腫瘍を小さくすることに成功
参照元 : カガクニュース隊
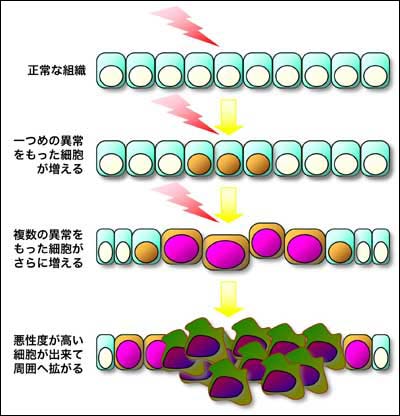
<細胞が癌化する仕組み>
Multiple boosts for cancer immunotherapy
参照元 : Science
2014年11月29日
がん細胞の腫瘍がなかなか小さくならないのは、免疫機能によって自然治癒しないからであると考えられてきたが、免疫細胞によって悪性細胞を治療することができる可能性が最新研究によって示唆された。
もしもそれが現実のものとなれば、がん細胞をコントロールすることが可能になり、より長期的な治療や、延命措置が可能になると言われている。
通常、腫瘍はT細胞を、CTLA4やPD-1といった免疫細胞によって無効化してしまうのだが、ある抗体を用いることによって、このCTLA4やPD-1をブロックし、T細胞に腫瘍を攻撃させることに成功した。
これは"治療"という言葉を使えるようなことではないにせよ、長期的な病気の管理や延命措置としては十分な可能性があると研究者は語っている。
こうした免疫による治療方法は、まだ一部のがんにしか効果が見られておらず、研究者は何が理由となって効果が現れる患者とそうでない患者がいるのかについて、引き続き調査を続ける見込みである。
ただ、いずれにせよ、免疫システムががん細胞を敵とみなすことができるというのは大きな発見であり、今後の進展次第では、本当にがんは恐ることのない病になるかもしれない。
参照元 : カガクニュース隊
<細胞が癌化する仕組み>

Multiple boosts for cancer immunotherapy
26 November 2014 3:30 pm
Tumors persist and grow in part because they squelch the immune system, but researchers have recently turned the tables with treatments that prompt immune cells to hunt down malignant cells. The strategy is effective only in some patients, however, and so far has been shown to work in just a few cancer types. But studies online today in Nature reveal how one kind of immunotherapy, so-called immune checkpoint inhibitors, can be targeted to new cancers—and how doctors can single out the patients who are most likely to benefit from these drugs.
“As a unit, these papers fill out many of the gaps in our understanding” about these cancer immunotherapies, says Jedd Wolchok, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City who wasn’t connected to the studies.
Tumors can suppress the cytotoxic T cells that would normally attack them by activating two immune cell surface receptors , CTLA4 and PD-1. But blocking CTLA4 and PD-1 with antibodies can unleash the T cells. Several clinical trials of patients with incurable cancers have shown dramatic effects from these antibodies. “What we are seeing is long-term disease control,” Wolchok says. “In some cases, people are living long enough to die from another cause.”
Researchers shy away from the c-word—cure—but they say that these immune checkpoint inhibitors could ultimately transform cancer into a manageable disease more like diabetes or HIV infection. “The immune system may be able to keep the tumor in check, even if it doesn’t eliminate every last cell,” says cancer immunologist Drew Pardoll of Johns Hopkins University School of Medicine in Baltimore, Maryland. Prospects like that led Science to anoint cancer immunotherapy as its Breakthrough of the Year for 2013.
Yet so far, published studies have confirmed that the checkpoint inhibitors work only in kidney cancer, melanoma, and lung cancer. And even in those cancers, usually less than half of patients benefit.
In one of the new studies, a team led by oncologist Thomas Powles of Queen Mary University of London tested a new antibody created by Genentech of South San Francisco, California, in a different cancer: a difficult-to-treat form of bladder cancer. Instead of blocking PD-1 on immune cells, the antibody disables PD-L1, a protein that cancer cells and some other cells display to stimulate PD-1 and inhibit T cells. The Genentech-funded study found that the antibody shrank bladder tumors in 26% of the patients. Therapy for this type of cancer has not advanced in 25 years, notes Hopkins medical oncologist Julie Brahmer, who wasn’t connected to the research. “This is truly groundbreaking,” she says.
In a second study, also funded by Genentech, translational oncologist Roy Herbst of the Yale School of Medicine and colleagues tested the antibody against several other kinds of incurable cancers. The patients in this trial “had exhausted one, two, three lines of treatment,” Herbst says, but he and his colleagues found that the antibody caused tumors to shrink in lung cancer, head and neck cancer, melanoma, and other tumor types. “It is unexpected and exciting to see a single drug having an impact on so many kinds of cancer,” says Suzanne Topalian, a cancer immunologist at Hopkins who didn’t take part in any of the studies.
Still, fewer than 20% of the patients in the Yale trial saw their tumors dwindle. “It’s a big conundrum to predict who will benefit from these treatments,” Herbst says. When he and his colleagues analyzed tumors from the trial’s patients, they found a potential explanation: variation in the amount of PD-L1 manufactured not by the cancer cells, but rather by the immune cells that had invaded the tumor. (The cancer cells may somehow compel immune cells to make the self-inhibiting molecules.) If these invading cells produced plenty of the protein, patients were more likely to respond to the antibody therapy. Testing PD-L1 levels in patients’ tumors might enable doctors to identify the people with the best chance of benefiting from the new antibody, Herbst says.
In a third Nature study, tumor immunologist and physician Antoni Ribas of the University of California, Los Angeles, and colleagues pinpointed another biomarker that might help improve the success rate of the immune-awakening antibodies. After poring over biopsy samples from the melanoma tumors of 46 clinical trial participants treated with a PD-1–blocking antibody, the researchers found that the best portent of treatment success was the abundance of cytotoxic T cells at the edge of the tumors. The more cells crowding into the edge of the tumor, the better.
To confirm their finding and gauge its usefulness, Ribas and colleagues then analyzed biopsy samples from melanoma patients in a clinical trial at another hospital. Using just this one feature, they correctly predicted how well 13 of the 15 patients would respond to the antibody therapy. “An assay that detects the presence of [cytotoxic T] cells in tumors could be the first decision point in the treatment of patients,” Ribas says.
Researchers are already looking at how to use the results of these studies to improve cancer immunotherapy, possibly by combining it with other types of treatments. “The next use will be in combination with other cancer drugs to get a greater impact,” Topalian says.
Posted in Biology, Health
参照元 : Science
























このブログにコメントするにはログインが必要です。
さんログアウト
この記事には許可ユーザしかコメントができません。